Supplier quality management is essential for maintaining product standards, minimizing risks, and building reliable partnerships. A central element in this process is effective quality system documentation, which not only fosters transparency and accountability but also helps align suppliers with your quality expectations.
In this guide, we’ll explore four essential documentation strategies that can elevate supplier quality management, ensuring consistency, efficiency, and continuous improvement.
The Power of Documentation in Supplier Quality Management
Managing supplier quality effectively requires more than just periodic evaluations—it requires a foundation of clear, well-maintained documentation. Quality system documentation serves as a roadmap for suppliers, defining expectations, and creating accountability. This documentation provides a consistent reference point that both suppliers and businesses can rely on to uphold quality standards. When implemented strategically, quality documentation can become a powerful tool for improving supplier quality management.
Here are the top 4 strategies:
1. Establishing Clear Quality Standards and Expectations
One of the most important steps in supplier quality management is setting clear and specific quality standards from the outset. Documentation plays a critical role in establishing these standards and preventing misunderstandings.
When quality standards are documented:
- Expectations are clear: Suppliers know exactly what is required of them, which minimizes confusion.
- Consistency is achieved: Detailed standards ensure that quality expectations are applied uniformly across all suppliers.
- Issues are minimized: By defining standards up front, businesses reduce the risk of quality issues that stem from miscommunication or misalignment.
Creating comprehensive documentation of quality standards, including product specifications, compliance requirements, and service expectations, provides suppliers with a clear framework to follow. This clarity fosters a more collaborative and productive relationship, helping both parties work toward a common quality goal.
2. Implementing Regular Documentation Reviews for Consistency
Regular review of quality documentation is essential to ensure that information remains accurate, relevant, and up-to-date. Periodic reviews of documentation help identify any discrepancies or gaps that may have developed over time, especially as supplier practices evolve or regulatory requirements change.
By conducting regular documentation audits:
- Gaps are identified: Regular audits reveal areas where documentation may be lacking or outdated.
- Continuous improvement is supported: Audits provide opportunities to refine and update documentation based on current quality standards and supplier performance.
- Consistency is reinforced: Ensuring that all suppliers are working with the most current standards maintains consistency across the supply chain.
An effective documentation audit process involves a systematic review of quality standards, compliance records, and performance benchmarks. By keeping documentation accurate and comprehensive, businesses can create a reliable reference for supplier quality management.
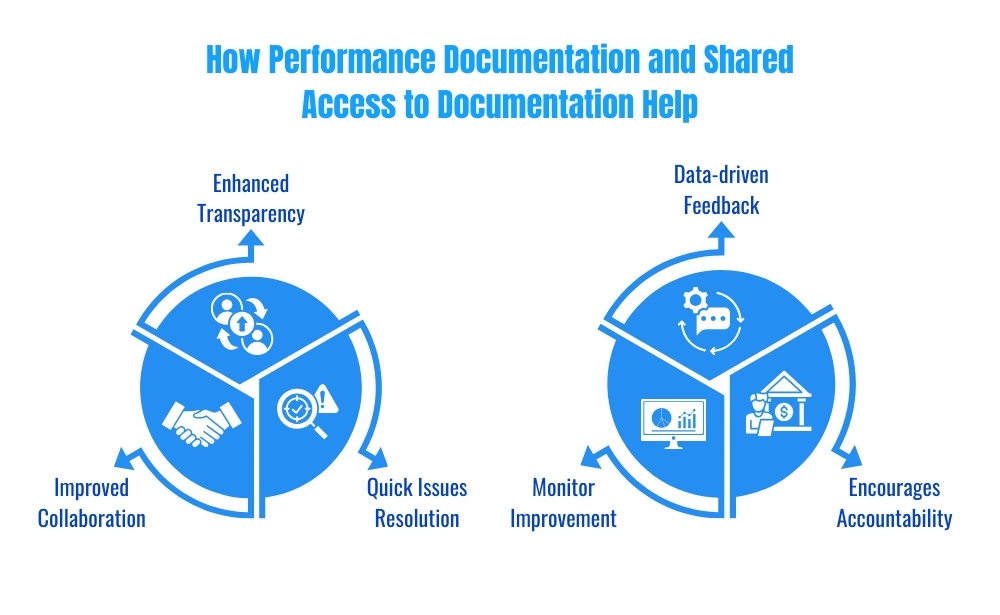
3. Using Performance Documentation to Foster Supplier Accountability
Tracking and documenting supplier performance is crucial for fostering accountability. When supplier quality data is meticulously documented, businesses have a factual basis for evaluating supplier reliability, responsiveness, and compliance with quality standards.
Performance documentation is valuable because:
- Supports data-driven feedback: Documented metrics provide a factual basis for feedback and discussions.
- Encourages accountability: Suppliers are more likely to adhere to standards when they know their performance is tracked.
- Helps monitor improvement: Historical data enables businesses to see trends in supplier performance over time.
By tracking metrics such as defect rates, delivery timelines, and compliance records, companies can use performance documentation to foster accountability. This data-driven approach ensures that feedback is objective, supporting constructive discussions that help suppliers align more closely with quality expectations.
4. Enabling Supplier Collaboration Through Shared Documentation
Supplier quality management is most effective when both parties can easily access relevant information. Shared documentation improves communication and collaboration, ensuring that suppliers have direct access to the quality standards they are expected to meet.
With shared access to documentation:
- Transparency is enhanced: Suppliers can view and understand the quality expectations set by the business.
- Collaboration is improved: Open access to documentation fosters a sense of partnership and shared responsibility.
- Issues are quickly addressed: Real-time access allows suppliers to address potential quality issues proactively.
Shared documentation platforms or portals where suppliers can view updated standards, performance requirements, and compliance guidelines create a streamlined process. This level of transparency not only builds trust but also empowers suppliers to take ownership of their quality management practices.
Conclusion
Strong documentation is more than just a regulatory requirement—it’s a strategic asset that can elevate supplier relationships and safeguard product quality. By focusing on clear quality standards, consistent audits, performance tracking, and collaborative documentation, businesses can foster a culture of accountability and continuous improvement with their suppliers.
Quality documentation not only streamlines processes but also establishes a foundation of trust and clarity, enabling suppliers to align seamlessly with a company’s quality objectives. Embracing these documentation strategies can transform supplier quality management, helping businesses achieve resilient, high-quality supply chains that are prepared to meet market demands.
Ready to take your supplier quality management to the next level? Explore how AsterDocs can help streamline and strengthen your quality documentation processes.