The nutraceutical supplements industry is experiencing rapid growth, fueled by increasing consumer interest in health and wellness. However, this growth also brings a complex array of regulations that companies must navigate to ensure their products are safe, effective, and compliant with global standards. As the industry evolves, the demand for more advanced compliance management strategies grows. This is where technology steps in—a game changer in how nutraceutical industries manage regulatory adherence.
The Challenges of Compliance in Nutraceutical Supplements
Compliance in the nutraceutical industry is a formidable task. Companies must adhere to a wide range of regulations set by bodies like the FDA, EFSA, and other international regulators. These rules cover everything from ingredient sourcing and manufacturing practices to labeling and marketing claims. Non-compliance can lead to severe penalties, product recalls, and lasting damage to a brand’s reputation.
Traditional compliance management methods, which often rely on manual processes and paper-based documentation, are becoming increasingly inadequate. The risk of human error coupled with the vast volume of documents required to prove compliance, presents significant challenges.
How Technology is Revolutionizing Compliance
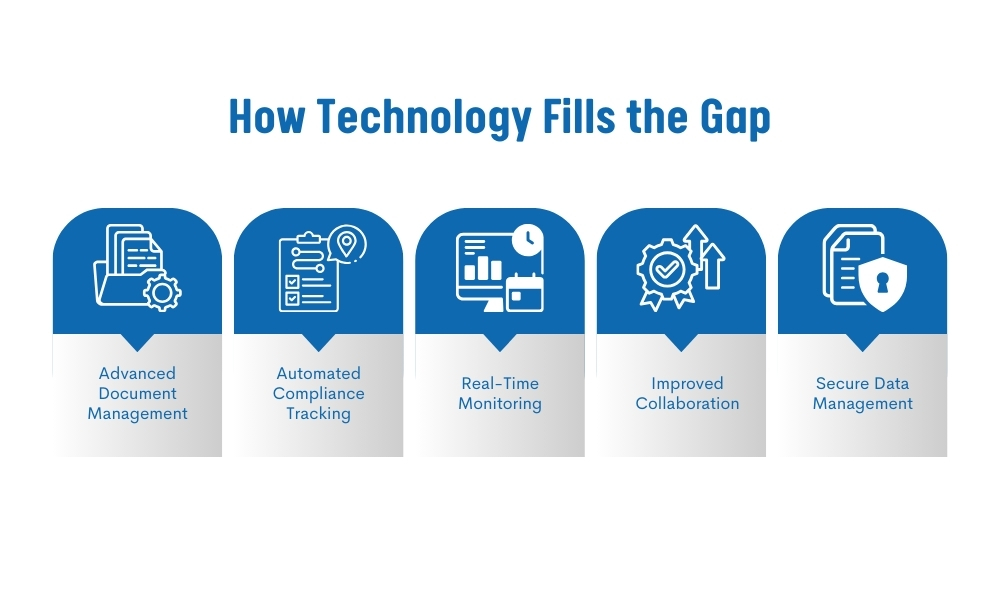
Fortunately, technology is stepping in to bridge the gap, offering innovative solutions that streamline the compliance process and reduce the burden on the nutraceutical supplement industries. Here’s how:
Advanced Document Management Systems:
One of the most significant technological advancements in compliance management is the emergence of advanced document management systems (DMS). These systems digitize and centralize all compliance-related documents, making it easier to store, retrieve, and manage the extensive data required for regulatory adherence.
Automated Compliance Tracking:
Technology allows for the automation of numerous compliance-related tasks. For instance, a robust DMS can automatically track regulatory changes and ensure that all documents are updated accordingly. This minimizes the risk of non-compliance due to outdated information.
Real-Time Monitoring and Reporting:
Advanced technology enables real-time monitoring of compliance activities. Companies can track their compliance status across multiple markets, identify potential risks, and generate detailed reports that can be easily shared with regulatory bodies.
Enhanced Collaboration:
Technology enhances collaboration between different departments within a company, as well as with external partners and suppliers. With a centralized DMS, all stakeholders can access the necessary documents and information, ensuring everyone is aligned on compliance requirements.
Data Security and Integrity:
In an industry where the integrity of compliance data is critical, technology offers enhanced security features. Advanced encryption, user authentication, and access controls ensure that only authorized personnel can access sensitive compliance documents.
The Future of Compliance in Nutraceutical Supplements
As the nutraceutical supplements industry continues to grow, the importance of compliance will only become more critical. Companies that fail to adapt to the evolving regulatory landscape risk falling behind their competitors and facing legal repercussions. The future of compliance in nutraceuticals lies in embracing technology.
With the continued advancement of AI, we can expect even more sophisticated compliance tools in the coming years. AI-powered systems could analyze vast amounts of data to predict regulatory changes, identify potential compliance issues before they arise, and offer automated solutions to address them.
Conclusion
The future of compliance in the nutraceutical supplements industry is undeniably tied to technology. By leveraging advanced document management systems and other technological innovations, nutraceutical companies can navigate the complex regulatory landscape with greater ease and confidence. As compliance requirements become more stringent, those who embrace these technologies will be better positioned to thrive in this competitive industry.
If you’re looking to streamline your compliance processes and stay ahead of regulatory changes, AsterDocs offers a cutting-edge document management software solution tailored specifically for the nutraceutical and dietary supplements industry. Explore how AsterDocs can help you achieve seamless regulatory adherence.