Ensuring product safety and regulatory compliance is paramount. To meet these demands, businesses must have a robust supplier quality management system in place. This system is crucial for maintaining product integrity and mitigating risks associated with suppliers. From sourcing raw materials to ensuring consistency in the final product, a well-established quality management system can make the difference between success and costly mistakes.
Supplier relationships are at the core of any nutraceutical business, and overlooking the quality management of these relationships can lead to significant issues. In this blog, we will explore why having a supplier quality management system is no longer an option but a necessity for nutraceutical manufacturers and how it helps with supplier risk mitigation.
What is a Supplier Quality Management System?
A supplier quality management system (SQMS) is a structured framework that helps businesses monitor and control the quality of goods and services provided by their suppliers. For nutraceutical manufacturers, this means keeping a close watch on everything from the sourcing of herbal ingredients to the delivery of final products. The system includes quality control measures, audit processes, and compliance checks to ensure that suppliers meet the necessary standards set by the nutraceutical industry.
In essence, a well-designed SQMS ensures that only high-quality, safe, and compliant materials enter the production line, thus maintaining the safety and efficacy of nutraceutical products.
Why Nutraceutical Manufacturers Need a Supplier Quality Management System
Nutraceutical manufacturers face unique challenges when it comes to sourcing ingredients and materials. With strict regulations, such as those set by the FDA and other global regulatory bodies, manufacturers need to ensure that their products are safe for consumers. This is where a supplier quality management system comes into play.
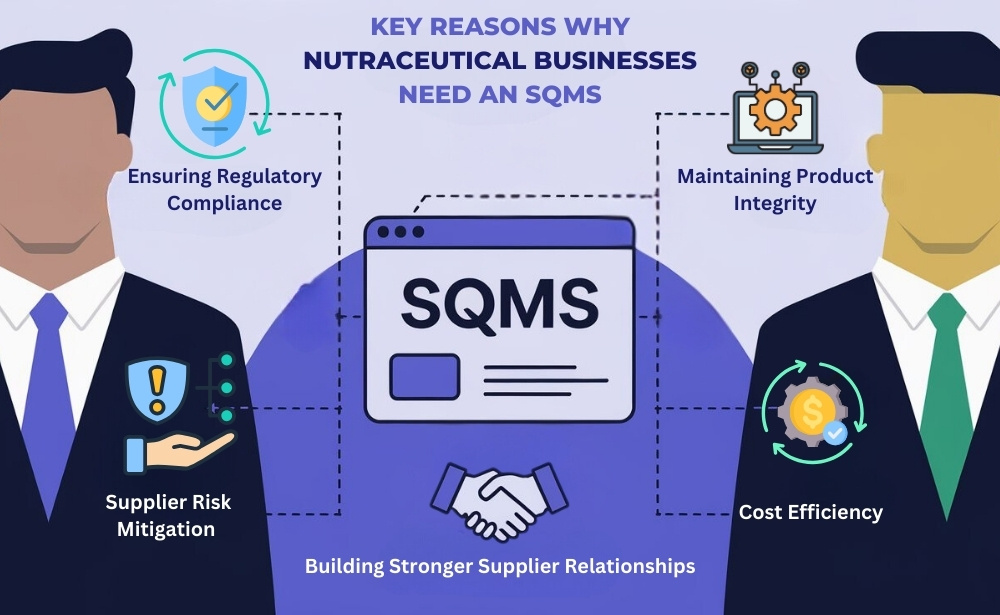
Here are key reasons why nutraceutical businesses should prioritize this system:
- Ensuring Regulatory Compliance Nutraceutical products are subject to strict regulations to ensure their safety and efficacy. A supplier quality management system helps manufacturers track and monitor compliance with industry standards, reducing the risk of non-compliance. With built-in compliance checks, SQMS ensures that suppliers adhere to these standards before materials enter the production process.
- Maintaining Product Integrity In the nutraceutical industry, product integrity is critical. Poor quality ingredients or mismanagement in the supply chain can affect the final product’s efficacy and safety. An SQMS helps in maintaining the quality of raw materials and ensures that each batch of product is consistent.
- Supplier Risk Mitigation Suppliers often operate across multiple locations and may not always be consistent in their processes. A supplier quality management system provides real-time visibility into supplier performance and helps mitigate risks associated with inconsistencies, delays, or quality failures. By implementing an SQMS, manufacturers can identify and address supplier-related risks before they impact production.
- Building Stronger Supplier Relationships Having a structured supplier quality management system promotes open communication and collaboration with suppliers. This helps build stronger, more transparent relationships, reducing misunderstandings and fostering long-term partnerships that benefit both parties.
- Cost Efficiency Addressing supplier issues before they escalate can save manufacturers a significant amount of money. Quality management systems help reduce the chances of recalls, regulatory penalties, or lost revenue due to poor product quality. This proactive approach to managing supplier risk allows for more efficient production and cost savings.
Key Elements of a Supplier Quality Management System
An effective supplier quality management system consists of several important components, each designed to ensure supplier performance and mitigate potential risks. Here’s a breakdown of key elements that nutraceutical manufacturers should focus on:
- Supplier Evaluation and Approval Evaluating suppliers is the first step in ensuring quality. The SQMS helps manufacturers assess potential suppliers based on criteria such as quality certifications, compliance history, and production capabilities. Approved suppliers are then continually monitored to ensure ongoing compliance.
- Quality Audits Regular quality audits are a crucial part of any SQMS. These audits verify that suppliers are following required quality procedures and meeting the standards set by the nutraceutical industry. Audits can cover everything from raw material sourcing to final product testing.
- Document Control Maintaining accurate records is essential for compliance and transparency. An SQMS offers centralized document control, ensuring that all necessary certifications, quality reports, and supplier agreements are stored and easily accessible.
- Performance Monitoring Ongoing monitoring of supplier performance is a must. By tracking key performance indicators (KPIs) such as delivery times, defect rates, and compliance with standards, manufacturers can quickly identify and address any issues that arise.
- Corrective and Preventive Actions (CAPA) The SQMS also supports corrective and preventive action plans when non-compliance or quality issues are detected. CAPA helps manufacturers resolve supplier issues swiftly while preventing future occurrences.
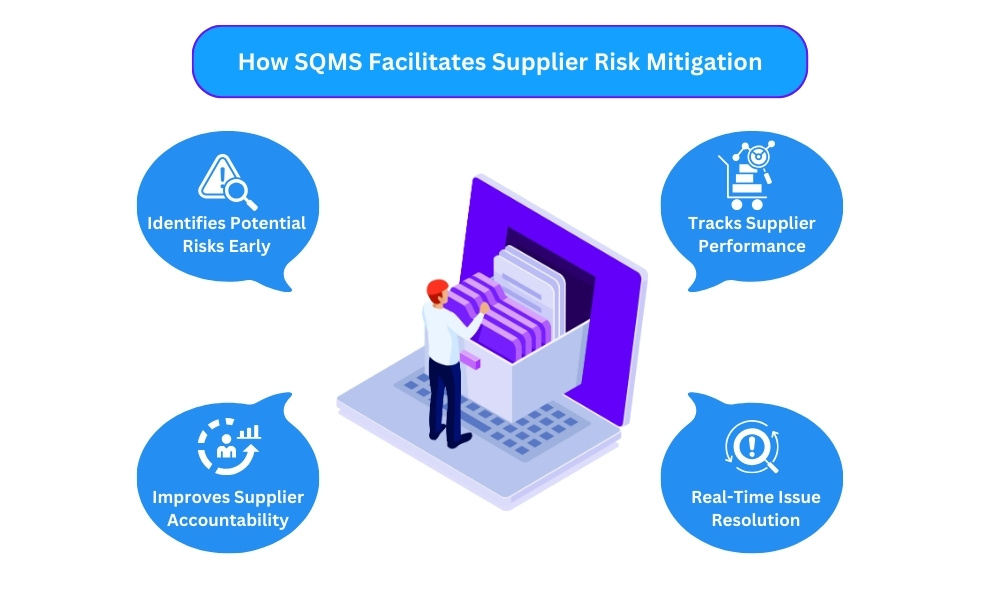
How SQMS Facilitates Supplier Risk Mitigation
Supplier risk is a critical concern for nutraceutical manufacturers, as disruptions or failures in the supply chain can have serious consequences. By integrating a supplier quality management system, businesses can take a more proactive approach to supplier risk mitigation.
- Identifying Potential Risks Early With a well-implemented SQMS, manufacturers can assess potential risks associated with each supplier early on. This allows them to take preventive measures, such as increasing quality checks or seeking alternative suppliers before the risks affect production.
- Tracking Supplier Performance Continuous monitoring of suppliers helps identify patterns or trends that could indicate future risks. Whether it’s late deliveries, inconsistent quality, or failure to comply with regulations, tracking supplier performance is essential for effective risk mitigation.
- Real-Time Issue Resolution One of the biggest advantages of a supplier quality management system is its ability to flag issues in real-time. Whether a batch of ingredients fails a quality check or there’s a delay in the supply chain, the SQMS enables quick resolution, reducing the impact on production timelines.
- Improving Supplier Accountability By maintaining detailed audit trails and compliance records, the SQMS ensures that suppliers are held accountable for their performance. This helps in building a culture of continuous improvement, where both the manufacturer and the supplier work together to minimize risks and enhance quality.
Conclusion:
In an industry where product quality and regulatory compliance are non-negotiable, implementing a supplier quality management system is essential for nutraceutical manufacturers. Not only does it ensure the quality and safety of your products, but it also plays a vital role in supplier risk mitigation.
By taking control of supplier relationships and ensuring that quality standards are consistently met, nutraceutical companies can operate with greater confidence, minimize risks, and stay ahead of the competition. For more details, visit Asterdocs now.