In recent years, the Food Safety Modernization Act (FSMA) has made significant changes to how food and dietary supplement manufacturers approach food safety. With tighter regulations and a focus on preventing contamination before it occurs, nutraceutical manufacturers are facing increasing pressure to meet FSMA compliance requirements. These regulations not only impact the way products are produced but also how they are managed throughout the supply chain. As we approach 2025, it is essential for nutraceutical companies to understand how FSMA compliance affects their operations and what steps they can take to stay ahead of the curve.
This blog will break down the essential aspects of food safety modernization act compliance and provide actionable insights for nutraceutical manufacturers to navigate the FSMA regulations effectively. We will look at the key changes under FSMA, their implications for the nutraceutical industry, and the role technology plays in ensuring compliance.
Key FSMA Compliance Requirements for Nutraceutical Manufacturers
The Food Safety Modernization Act (FSMA) was signed into law to ensure that food safety regulations are modernized to better prevent contamination and protect public health. While it covers the entire food industry, nutraceutical companies must comply with specific FSMA compliance requirements tailored to their unique needs. Understanding these regulations is critical to maintaining product safety and compliance.
Key FSMA Compliance Requirements for Nutraceutical Manufacturers:
- Hazard Analysis and Risk-Based Preventive Controls (HARPC):
Under FSMA, manufacturers are required to assess potential hazards in their production processes and implement preventive controls to mitigate those risks. For nutraceuticals, this means identifying hazards such as contamination risks in raw materials or during manufacturing and taking necessary precautions to prevent them. - Supply Chain Controls:
FSMA requires companies to ensure that their suppliers are also adhering to safety standards. Nutraceutical companies need to evaluate and monitor their suppliers to ensure the ingredients they receive are compliant with food safety regulations. This includes conducting supplier audits and ensuring proper documentation is maintained. - Traceability and Recordkeeping:
FSMA emphasizes the importance of recordkeeping and traceability. Nutraceutical manufacturers must maintain detailed records of all processes, including production, distribution, and handling. This is crucial for being able to trace any potential issues in case of a recall or safety incident. - Food Safety Plans:
Companies must develop and implement a food safety plan that addresses hazard analysis, preventive controls, and the monitoring of compliance. This plan is essential for maintaining ongoing FSMA compliance and ensuring that the company’s operations are always aligned with safety standards.
These FSMA compliance regulations are designed to minimize risk and ensure the quality and safety of nutraceutical products. Compliance may seem overwhelming, but understanding these fundamental requirements is the first step in ensuring that your company remains compliant and avoids penalties.

The Impact of FSMA on Nutraceutical Manufacturers’ Operations
The implementation of FSMA compliance requirements affects nearly every aspect of nutraceutical manufacturing operations. From production processes to documentation and recordkeeping, FSMA introduces new challenges for manufacturers to manage.
How FSMA Affects Nutraceutical Manufacturers:
- Increased Operational Costs:
Compliance with FSMA may require significant investments in upgrading facilities, implementing preventive controls, and adopting new technologies to streamline documentation and reporting. While the costs of compliance may seem high initially, the long-term benefits of avoiding penalties and ensuring product safety far outweigh the costs. - More Detailed Recordkeeping:
FSMA requires businesses to maintain extensive documentation regarding food safety practices. Nutraceutical manufacturers will need to create and maintain detailed records for each step of the production process, including ingredient sourcing, production protocols, and quality control measures. The need for clear and consistent recordkeeping creates a more formalized system of operations. - Stronger Supplier Management:
FSMA requires manufacturers to conduct regular audits of their suppliers, ensuring that the ingredients and materials used in their products meet the required food safety standards. This increases the burden on nutraceutical companies to manage and monitor their supply chains more closely. Supplier compliance with food safety standards is now essential for maintaining overall product safety. - Adoption of New Technology:
Nutraceutical manufacturers must consider implementing advanced technologies, such as automated recordkeeping and data tracking systems, to ensure compliance. This can involve adopting document management systems, using AI-powered solutions to track compliance data, and integrating systems for real-time monitoring of safety standards throughout production.
The operational impact of FSMA compliance on nutraceutical companies is significant, but with the right tools and strategies in place, manufacturers can meet these challenges head-on. By adopting modern technology and prioritizing food safety at every level of the production process, companies can ensure their long-term compliance and success in the market.

Technology’s Role in Ensuring FSMA Compliance for Nutraceutical Manufacturers
Incorporating automation technologies into operations is becoming a must for ensuring FSMA compliance in the nutraceutical industry. These tools provide manufacturers with real-time data, automate recordkeeping, and help manage their supply chain compliance more effectively.
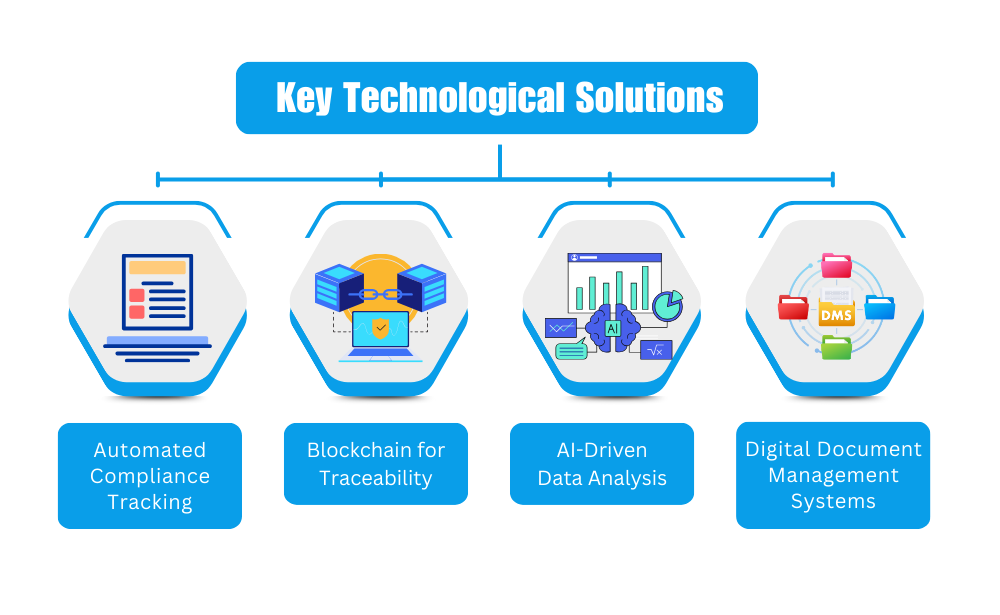
Key Technological Solutions for FSMA Compliance:
- Automated Compliance Tracking:
Automating the process of compliance tracking can help nutraceutical companies reduce the risk of human error and streamline their operations. With automated systems, manufacturers can track the status of their food safety modernization act compliance in real-time and generate reports with ease, ensuring that all necessary documentation is up-to-date and readily available for audits. - Blockchain for Traceability:
Blockchain technology offers a way to maintain tamper-proof, transparent records of the entire supply chain. This is particularly valuable in the nutraceutical industry, where traceability is essential for ensuring the safety and integrity of ingredients. Blockchain allows manufacturers to verify that their suppliers meet FSMA compliance requirements, improving overall trust and transparency. - AI-Driven Data Analysis:
AI can be used to analyze data from various sources, identifying potential risks and ensuring that preventive measures are implemented across the supply chain. By using AI to assess and predict risks, manufacturers can ensure that they’re meeting FSMA standards and maintaining product safety. - Digital Document Management Systems:
With document management systems, manufacturers can store, track, and share compliance-related documents in a centralized location. These systems make it easy to access historical data, manage version control, and ensure that all relevant stakeholders are up to date with compliance requirements.
By leveraging technology, nutraceutical manufacturers can simplify the process of FSMA compliance and focus on the quality and safety of their products, all while reducing the administrative burden and improving operational efficiency.
Conclusion
As FSMA compliance becomes increasingly important in 2025, nutraceutical manufacturers need to be proactive in ensuring that their operations meet the required standards. The Food Safety Modernization Act requires companies to implement preventive controls, maintain detailed records, and ensure traceability of products throughout the supply chain. While the compliance process may seem daunting, adopting advanced technologies can streamline the process and reduce the burden on manufacturers.
By prioritizing food safety and compliance and integrating the right tools, nutraceutical companies can meet the evolving requirements of FSMA while continuing to deliver safe, high-quality products to their customers.
Is your business ready for FSMA compliance in 2025? Start adopting smart digital tools to streamline your compliance process today. Reach out to us to learn how our platform can help you stay compliant and competitive in the nutraceutical industry.












