As the demand for nutraceutical products continues to grow, so does the need for high-quality suppliers to meet the increasing demand.
However, identifying and qualifying reliable nutraceutical suppliers can be a challenging process, given the complexity and variability of the industry.
From regulatory compliance to quality assurance, there are numerous hurdles that companies must navigate to ensure that they are working with reputable and trustworthy suppliers.
In this blog post, we will explore some of the key challenges companies face when qualifying nutraceutical suppliers and some tips and best practices to help overcome these obstacles.
Whether you are a manufacturer, distributor, or retailer of nutraceutical products, these insights can help you streamline your supplier qualification process.
It ensures that you are partnering with suppliers that meet your high standards for quality and reliability.
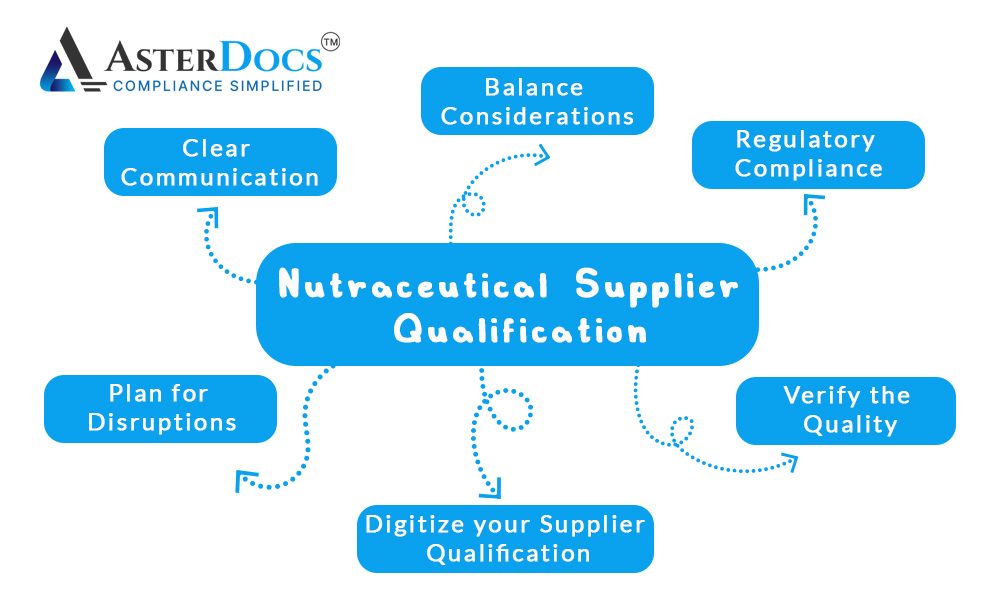
Establish Clear Communication with Suppliers:
Clear Communication is key regarding a lack of transparency. Ask your suppliers detailed questions about their ingredients and manufacturing processes.
Don’t hesitate to request documentation or visit their facilities to ensure their practices align with your standards. By establishing a clear line of communication and being transparent about your expectations, you can build a stronger relationship with your suppliers and ensure that you receive high-quality ingredients.
Verify the Quality of Ingredients:
In addition to establishing clear communication and verifying the quality of the ingredients you receive is important. Third-party testing is one way to accomplish this.
Using an independent testing laboratory, you can objectively evaluate the quality and purity of the ingredients.
You may also consider working with suppliers already certified by third-party organizations, such as the Non-GMO Project or the Natural Products Association.
Ensure Regulatory Compliance:
Ensuring regulatory compliance can be challenging, particularly when working with suppliers in different countries. However, you can ensure that your suppliers comply with relevant regulations.
One approach is to work with suppliers with a strong regulatory compliance track record. Another approach is to communicate your expectations around compliance from the outset of your relationship.
You may also want to consider hiring a consultant specializing in regulatory compliance to help you navigate the complex landscape of regulations.
Plan for Supply Chain Disruptions:
Supply chain disruptions are a fact of life in the nutraceutical industry.
However, there are steps you can take to minimize their impact. One approach is to work with suppliers with multiple ingredient sources. This can help ensure a reliable supply of high-quality ingredients even if one source becomes unavailable.
You may also want to consider building up your inventory of key ingredients to ensure a buffer in case of a disruption.
Balance Cost Considerations with Quality and Safety Considerations:
Finally, balancing cost and safety considerations is important. While cost is always a factor; it’s important to remember that high-quality ingredients and regulatory compliance come with a price tag.
Be wary of suppliers who offer significantly lower prices than their competitors, as they may not be able to deliver the same level of quality and safety. Look for suppliers who offer competitive pricing and have a strong track record of quality and safety.
In conclusion,
Qualifying nutraceutical suppliers can be a challenging process. Still, it’s essential to ensure that you provide high-quality, safe products to your customers.
By following these tips and best practices, you can overcome the challenges involved in supplier qualification and establish long-term, loyal relationships with suppliers that meet your high quality, safety, and regulatory compliance standards.
Remember to establish clear communication, verify the quality of ingredients, ensure regulatory compliance, plan for supply chain disruptions, and balance cost considerations with quality and safety considerations.
By taking these steps, you can streamline your supplier qualification process and ensure that you’re partnering with suppliers that can help you meet the growing demand for nutraceutical products.
Is it possible to digitize your supplier qualification procedures?
To prioritize supplier risk management, digitizing supplier management and contracts is essential. Even though many organizations have recognized this, some still require a more modern solution.
If your current system is manual, ensuring that your suppliers and external vendors prioritize regulatory compliance and product quality can be difficult.
The AsterDocs supplier quality management system offers a specialized solution that allows you to keep track of all supplier quality data and documentation in one location, resolving this issue.
This system allows you to organize supplier qualification and audits, link suppliers and supplier risk, and get a clear documentation overview. The software solution also streamlines supplier qualification and corrective action requests in a single location.
Supplier collaboration can be increased by implementing specialized software. Software that provides real-time insights, data-driven decision-making capabilities, and risks can be mitigated. This can provide the agility required to create a strategic path forward.