In the ever-evolving regulatory landscape of nutraceuticals, maintaining a robust and organized system for compliance documentation is critical. This includes a vast array of documents like policies, procedures, regulations, training materials, and more. However, managing these documents manually can quickly become a tangled mess, leading to inefficiencies, version control issues, and difficulties during audits.
Challenges of Manual Compliance Documentation Management:
-
Scattered Documents: Documents stored in various physical locations (file cabinets, employee desktops) or digital formats (email inboxes, personal drives) become difficult to locate and manage efficiently.
-
Version Control Issues: Multiple versions of the same document floating around can create confusion and the risk of using outdated information.
-
Limited Accessibility: Restricted access for authorized personnel can hinder collaboration and timely updates to critical documents.
-
Audit Trail Challenges: Manually tracking changes made to documents over time creates a significant burden during audit preparation, making it difficult to demonstrate a clear history of compliance.
Benefits of Centralized Compliance Documentation:
-
Enhanced Organization: Centralize all compliance documents in a single, secure, and easily accessible location. This eliminates the need to search through various folders or inboxes to find what you need.
-
Improved Version Control: Implement a system for tracking and managing different versions of documents with clear version history. This ensures everyone is working with the latest and most accurate information.
-
Streamlined Access: Define access levels for different user roles within your organization. This ensures only authorized personnel can view, edit, or download specific documents, promoting security and compliance.
-
Simplified Audit Preparation: Maintain a clear audit trail with documented changes and version history for each document. This allows you to easily demonstrate a well-organized and compliant documentation system to regulatory bodies during audits.
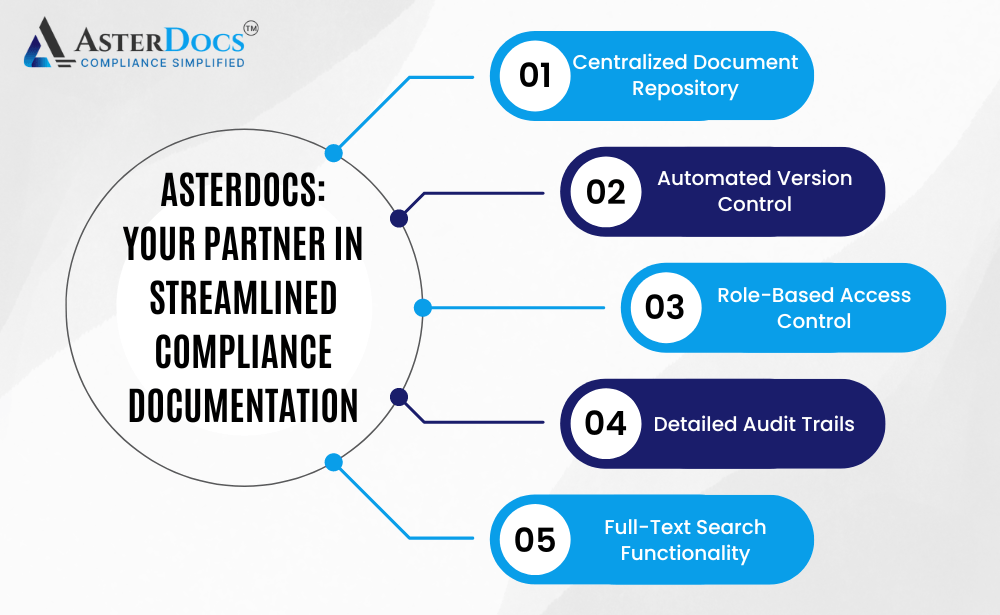
Asterdocs: Your Partner in Streamlined Compliance Documentation
Asterdocs empowers you to overcome the challenges of manual document management and achieve efficient compliance documentation with a comprehensive solution that includes:
-
Centralized Document Repository: Store all your compliance documents in a secure and centralized cloud platform. This provides easy access for authorized personnel from any location with an internet connection.
-
Automated Version Control: Track and manage different versions of documents with clear version history. Asterdocs automatically alerts users of new versions, ensuring everyone is working with the latest information.
-
Role-Based Access Control: Define user roles and assign access levels for each role. This ensures only authorized personnel can view, edit, or download specific documents, promoting data security and compliance.
-
Detailed Audit Trails: Maintain a complete and auditable record of all changes made to documents, including timestamps, usernames, and specific edits. This simplifies audit preparation and demonstrates a commitment to ongoing compliance.
-
Full-Text Search Functionality: Easily locate specific documents or information within documents using a powerful search function. This saves time and frustration when searching for critical information.
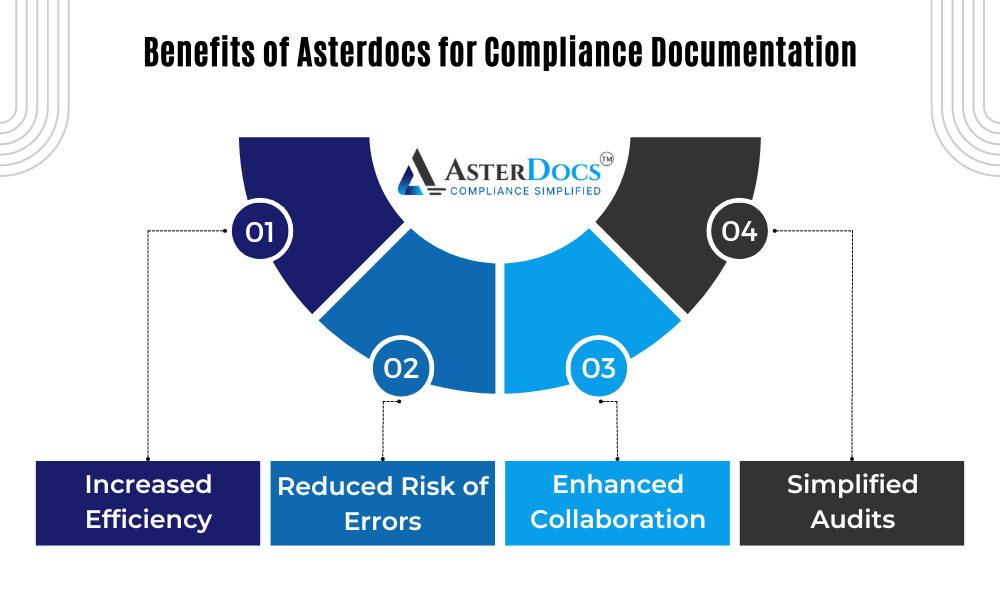
Benefits of Asterdocs for Compliance Documentation:
-
Increased Efficiency: Streamline document management processes, reduce time spent searching for documents, and improve overall team productivity.
-
Reduced Risk of Errors: Minimize the risk of using outdated information or encountering version control issues, ensuring everyone has access to the latest and most accurate documents.
-
Enhanced Collaboration: Facilitate easy access and collaboration on compliance documents for authorized personnel, promoting a culture of compliance awareness.
-
Simplified Audits: Demonstrate a clear and well-organized compliance documentation system to regulatory bodies with ease, reducing audit stress and potential delays.
Conclusion:
Effective compliance documentation management is no longer a luxury in the nutraceutical industry; it’s a necessity. By adopting a centralized and automated approach with Asterdocs, you can conquer the chaos of compliance documentation, ensure ongoing compliance, and empower your team to focus on what matters most – developing and delivering high-quality nutraceutical products.
Ready to streamline your compliance documentation and achieve peace of mind? Contact Asterdocs today to learn how our solutions can empower your business!