In industries like manufacturing, healthcare, pharmaceuticals, and nutraceuticals, maintaining outstanding compliance document control is not just a best practice—it’s a necessity. With stringent regulations in place to protect consumers and ensure product quality, the management of business documents must be meticulous and well-organized. When companies fail to maintain proper document control, the consequences can be severe, ranging from compliance issues to costly legal battles.
This blog explores the importance of quality document control, particularly in industries that are tightly regulated, and how it helps businesses stay compliant with international standards. It also highlights the challenges companies face and how they can address them to improve efficiency and maintain industry standards.
What Are the Standards for Document Control?
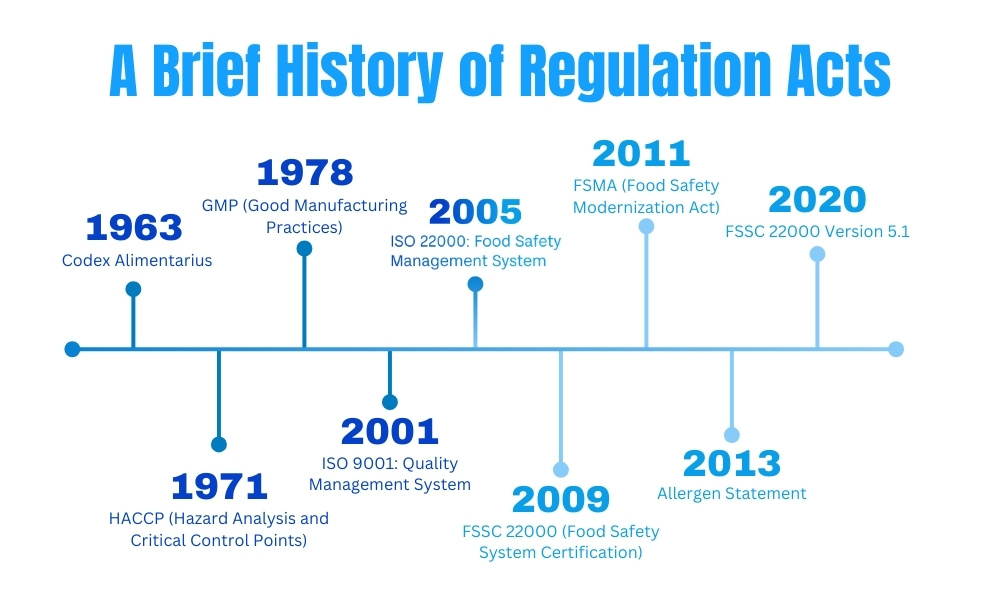
In highly regulated industries, adhering to international standards is crucial to ensuring that products and services meet safety and quality requirements. These standards are designed to protect both consumers and businesses by ensuring that companies operate with transparency, accuracy, and accountability.
Key International Standards for Document Control:
- ISO 9001: Quality Management Systems (QMS)
ISO 9001 is a globally recognized standard for quality management. It provides guidelines for maintaining quality standards in every aspect of business operations, from production processes to customer service. For document control, ISO 9001 requires businesses to maintain detailed records, track document changes, and ensure proper document approval and review procedures. The standard also requires companies to regularly audit their document control systems for compliance. - Good Manufacturing Practice (GMP):
GMP regulations ensure that products are consistently produced and controlled according to quality standards. In industries like pharmaceuticals and nutraceuticals, GMP mandates stringent documentation procedures to ensure that production processes are accurately recorded, ingredients are traceable, and products are made under conditions that guarantee their quality. GMP requires businesses to have clear documentation processes, such as batch records, inspection logs, and equipment maintenance records, to verify compliance. - FDA Regulations (e.g., 21 CFR Part 11 for Pharmaceuticals and Nutraceuticals):
For companies operating in the nutraceutical and pharmaceutical industries, FDA regulations are some of the most important guidelines to follow. The 21 CFR Part 11 rule, for example, dictates the use of electronic records and signatures in place of paper-based documentation. This regulation requires companies to maintain an audit trail for all documents related to product manufacturing, testing, and distribution to ensure compliance and traceability. The FDA mandates that all documents are accessible, accurate, and secure, and that they meet regulatory requirements for record-keeping and data integrity. - Hazard Analysis Critical Control Point (HACCP):
In the food and nutraceutical industries, HACCP is a system used to ensure food safety by controlling biological, chemical, and physical hazards. For document control, HACCP requires that businesses maintain records of critical control points during production, ingredient sourcing, and testing to ensure consumer safety. All documents related to the production and distribution of food and nutraceutical products must be accurately maintained to meet these standards.
Aligning Document Control with These Standards:
For businesses in regulated industries, aligning document control practices with international standards is essential to avoid costly mistakes, penalties, and legal complications. Companies should develop internal processes that ensure documents are updated, reviewed, and approved in accordance with these regulations. This includes using automated systems for document version control, approval workflows, and audit trails, which ensure full traceability and accountability.
Steps to Achieving Quality Document Control
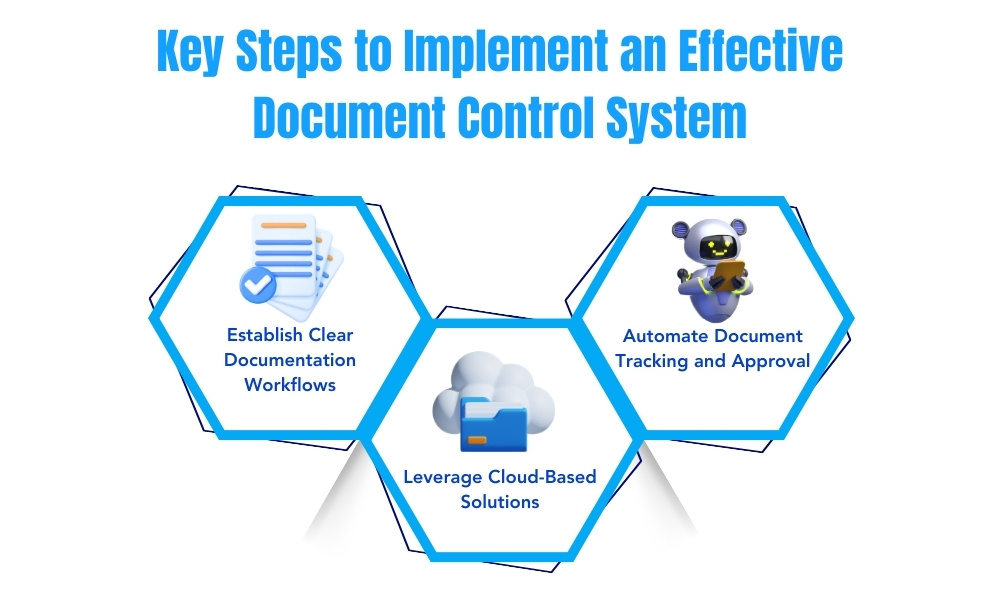
Achieving quality document control involves more than just storing documents in an organized manner. It requires well-established workflows, secure access, and regular audits. Here are some key steps companies can take to implement an effective compliance document control system:
1. Establish Clear Documentation Workflows
Document workflows should outline the processes for creating, reviewing, approving, and distributing documents. This includes assigning roles and responsibilities for each step in the process, such as:
- Document Creation: Who is responsible for creating new documents, such as Standard Operating Procedures (SOPs) or quality assurance (QA) records?
- Review and Approval: Who needs to review the document, and who gives final approval?
- Distribution and Access: Who can access the final version of the document, and how is it shared across teams?
Clear workflows prevent confusion and ensure that documents are created and handled according to regulatory requirements.
2. Leverage Cloud-Based Solutions for Easy Access and Collaboration
A cloud-based document management system (DMS) allows employees to access, collaborate on, and share documents securely from any location. These systems improve document visibility, reduce the risk of errors, and ensure that teams are working with the most up-to-date information. Cloud-based solutions also help with version control, as they automatically track changes made to documents, making it easier to identify who made changes and when.
3. Automate Document Tracking and Approval Processes
Automation reduces human error and ensures that documents move through the approval process more efficiently. With automated workflows, businesses can set up automatic notifications for document approvals, reviews, and updates. This not only speeds up the process but also ensures compliance with regulations that require timely reviews and approvals.
Automating document tracking also creates a clear audit trail that records every action taken on a document, making it easier to prove compliance during audits or inspections.
Real-World Challenges in Document Control
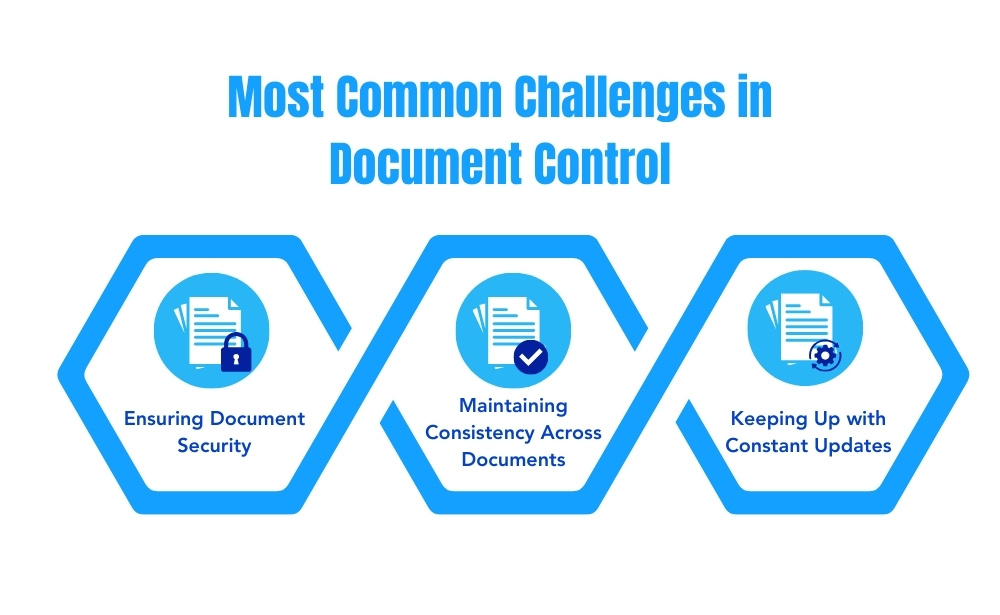
While implementing a quality document controller can improve efficiency and compliance, businesses face several challenges in maintaining organized, up-to-date records. Some of the most common challenges include:
1. Maintaining Consistency Across Documents
With multiple departments or teams handling various documents, maintaining consistency in terms of format, content, and updates can be difficult. Establishing clear document naming conventions, standard operating procedures, and version control rules can help reduce inconsistencies.
2. Ensuring Document Security
With the growing risk of cyber threats, it is essential to implement strong security measures to protect sensitive information. This includes setting up secure access protocols, encrypting sensitive documents, and ensuring compliance with data protection laws like GDPR.
3. Keeping Up with Constant Updates
As regulations evolve, businesses must continuously update their documentation to ensure it remains compliant. Regular audits and document reviews are essential to ensuring that all documents are current and accurate.
Solutions:
- Version Control Systems: Keep track of document revisions and ensure that only the latest version is in circulation.
- Integrated Document Management Systems (DMS): DMS platforms automate document storage, approval, and revision tracking, which simplifies document management and enhances security.
Conclusion
Quality document control is a critical component of maintaining industry standards, especially in regulated sectors like pharmaceuticals, healthcare, manufacturing, and nutraceuticals. Properly managed documents not only ensure compliance but also help improve operational efficiency, reduce risk, and foster collaboration across teams.
By adopting best practices such as clear documentation workflows, leveraging cloud-based systems, and automating tracking processes, businesses can ensure their documents remain accurate, accessible, and compliant with industry regulations.
To implement an effective document controller, you can explore solutions that provide security, transparency, and efficiency, like AsterDocs. If you want to improve your compliance document management processes, visit our website for more insights and resources.