Quality document control is crucial for the nutraceutical industry. Ensuring that every document related to compliance, quality standards, and ingredient safety is accurate and accessible helps build trust and avoid regulatory issues. However, managing quality documentation comes with its own set of challenges, especially in an industry as regulated as nutraceuticals. Here, we’ll dive into some of the most common challenges and practical ways to overcome them.
Why Quality Document Control is Essential for the Nutraceutical Industry
In the nutraceutical industry, regulatory compliance is not just a requirement—it’s a cornerstone of business success. From ensuring the safety of ingredients to meeting international standards, quality document control is essential for maintaining product integrity and protecting consumer health.
Proper document control helps businesses:
- Maintain Compliance: Meet FDA, GMP, and other regulatory standards.
- Ensure Traceability: Keep a detailed history of every document to maintain transparency.
- Build Consumer Trust: Demonstrate commitment to quality and compliance, which is crucial in health-related industries.
A solid document control system is the backbone that supports these goals, providing a reliable framework for managing essential documentation.
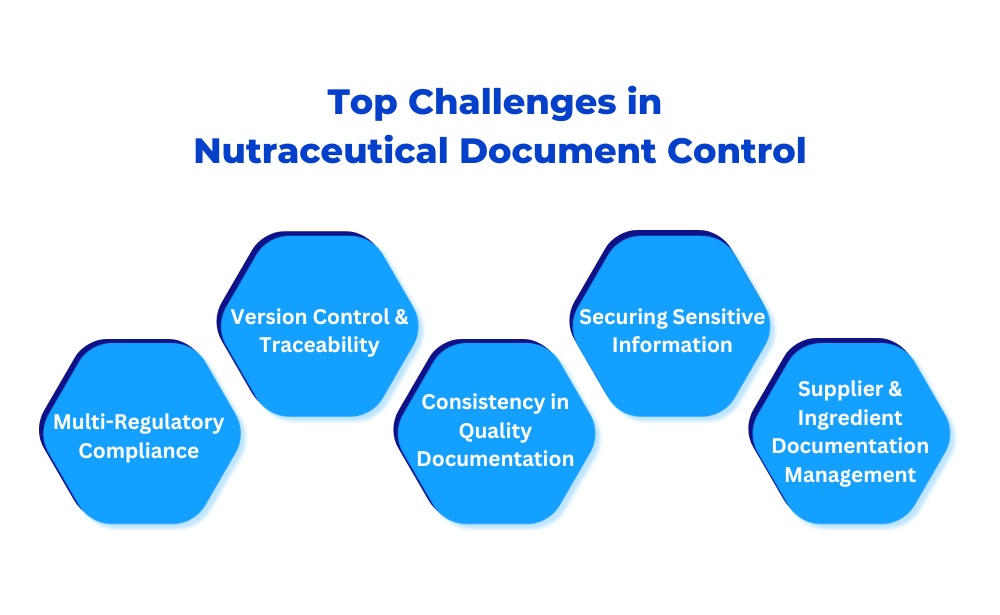
Top Challenges in Nutraceutical Quality Document Control
1. Managing Compliance Across Multiple Regulatory Bodies
The nutraceutical industry is governed by several regulatory bodies, such as the FDA, GMP, and ISO. Each has its own set of requirements, and often, these standards overlap or even conflict. This complexity makes it challenging to maintain documentation that meets all regulatory requirements simultaneously.
Solution:
- Implement a centralized compliance document system that allows easy access to documents organized by regulatory standards.
- Regularly audit your documents to ensure they align with the latest regulations, reducing the risk of non-compliance.
2. Document Version Control and Traceability
Tracking document versions can be a challenge, especially when multiple team members are involved in editing and approving documents. Without proper version control, outdated documents might be used, leading to errors and compliance risks.
Solution:
- Use a document management system that includes automated version control and audit trails. This ensures that only the latest, approved versions of documents are accessible for use.
- Set up alerts for any changes to critical documents so that all stakeholders are informed of updates in real-time.
3. Ensuring Consistency in Quality System Documentation
Quality system documentation must be consistent in structure, terminology, and format to prevent confusion and ensure everyone is aligned on the standards. However, maintaining this level of consistency across different departments and teams is often difficult.
Solution:
- Create standardized templates for all types of quality documents to maintain a uniform structure.
- Train teams on proper document formatting and terminology to reduce inconsistencies.
4. Securing Sensitive Information
The nutraceutical industry handles sensitive information, from ingredient sources to proprietary formulations. Ensuring that only authorized personnel have access to these documents is crucial to protect the company’s intellectual property.
Solution:
- Use role-based access controls within your document management system, allowing only authorized users to view or edit sensitive documents.
- Implement audit trails to track who accesses each document, adding an additional layer of security and accountability.
5. Managing Supplier and Ingredient Documentation
Nutraceutical companies often rely on a global network of suppliers, each providing essential compliance documents like Certificates of Analysis (COA), Material Safety Data Sheets (MSDS), and country of origin certificates. Collecting and verifying these documents can be time-consuming and prone to error.
Solution:
- Establish a document management process dedicated to supplier documentation. This ensures that all required documents are obtained, organized, and stored correctly.
- Set up automated reminders to prompt suppliers to provide updated compliance documents, reducing the risk of non-compliance.
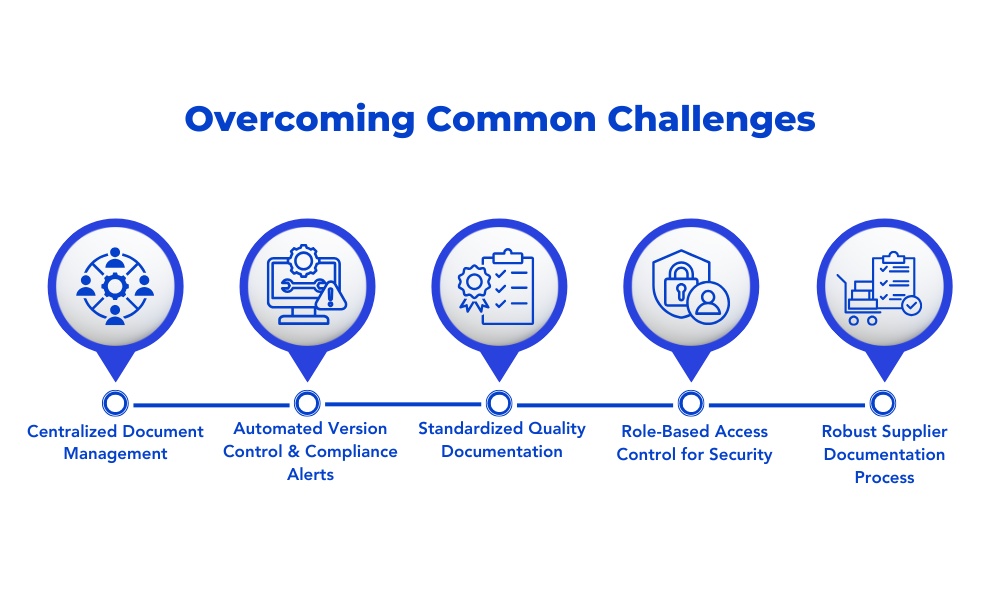
How to Overcome Common Challenges in Quality Document Control
Implementing a Centralized Document Management System
A centralized system provides one secure location for all documents, enabling easy retrieval and consistent organization. It simplifies compliance tracking and ensures that every team has access to the latest versions of essential documents.
Benefits:
- Reduces time spent searching for documents.
- Enhances document accuracy by minimizing the risk of outdated files being used.
- Improves collaboration across departments, with everyone working from the same platform.
Leveraging Automation for Version Control and Compliance Updates
Automation can significantly reduce the manual workload involved in tracking document versions and staying updated with regulatory changes. Automated notifications can alert team members to updates or required approvals, ensuring a smooth compliance process.
Benefits:
- Saves time and reduces human error.
- Ensures that all team members are using the latest document versions.
- Enhances readiness for audits by maintaining a comprehensive record of document changes.
Standardizing Quality System Documentation
Standardized documentation brings consistency across departments, making it easier to maintain a high level of quality control. Templates and pre-approved formats ensure that every document meets the same standards.
Benefits:
- Reduces discrepancies and misunderstandings.
- Simplifies training and onboarding by providing a consistent structure.
- Increases efficiency by providing teams with ready-to-use formats.
Securing Digital Documentation with Role-Based Access Control
Digital security is paramount in safeguarding sensitive information. Role-based access ensures that only the right people can access critical documents, reducing the risk of unauthorized access.
Benefits:
- Protects proprietary information and company IP.
- Adds accountability by tracking who accesses each document.
- Maintains a secure audit trail for sensitive records.
Establishing Strong Supplier Documentation Processes
A well-defined process for managing supplier documentation can simplify compliance and build trust in your supply chain. By setting up a system for verifying supplier credentials and compliance, you reduce the risk of issues further down the line.
Benefits:
- Ensures that suppliers meet industry and company standards.
- Reduces compliance risks associated with ingredient quality and safety.
- Builds stronger, more reliable supplier relationships.
Best Practices for Effective Quality Document Control in Nutraceuticals
To maximize the benefits of a quality document control system, follow these best practices:
- Regularly review and update documents to keep pace with regulatory changes.
- Conduct internal audits to ensure all documents align with compliance requirements.
- Train staff on the importance of document control and the specific processes involved.
- Maintain a centralized, organized document library that’s easy to navigate and manage.
Conclusion
Effective quality document control is the backbone of compliance and quality assurance in the nutraceutical industry. By understanding and addressing the common challenges, nutraceutical companies can build a stronger foundation for regulatory compliance and quality management. Implementing robust solutions—such as centralized document management, automation, and secure access controls—can significantly improve your document control processes, helping you stay compliant, consistent, and audit-ready.
For a comprehensive solution tailored to the nutraceutical industry, consider using AsterDocs to streamline your quality document control and elevate your compliance efforts.













