For businesses in regulated industries like nutraceuticals, pharmaceuticals, and food manufacturing, maintaining supplier compliance is essential. Ensuring that suppliers consistently meet quality standards, follow regulatory requirements, and adhere to best practices can be complex. However, with an effective quality document control system, businesses can streamline their supplier management processes, reduce compliance risks, and achieve higher operational standards.
Quality document control is more than just managing documents; it involves ensuring that every document related to supplier compliance is updated, accessible, and accurately tracks the performance of each supplier. By strategically implementing quality document control, companies can make compliance easier and more reliable. This blog will explore how a robust quality document control system supports supplier quality management and supplier compliance document requirements.
Why Quality Document Control is Essential for Supplier Compliance
In industries with strict regulatory requirements, supplier compliance isn’t just a formality—it’s critical for maintaining product quality and safety. According to research by McKinsey, companies with efficient supplier compliance systems report a 20% reduction in supply chain disruptions. The benefits of quality document control are clear:
- Streamlined compliance management: With organized documentation, compliance management is easier and more transparent, reducing the time and resources required.
- Improved supplier relationships: Effective document control systems build trust with suppliers, showing them that compliance is a priority.
- Enhanced product safety: By ensuring suppliers meet compliance standards, businesses protect their products and end consumers.
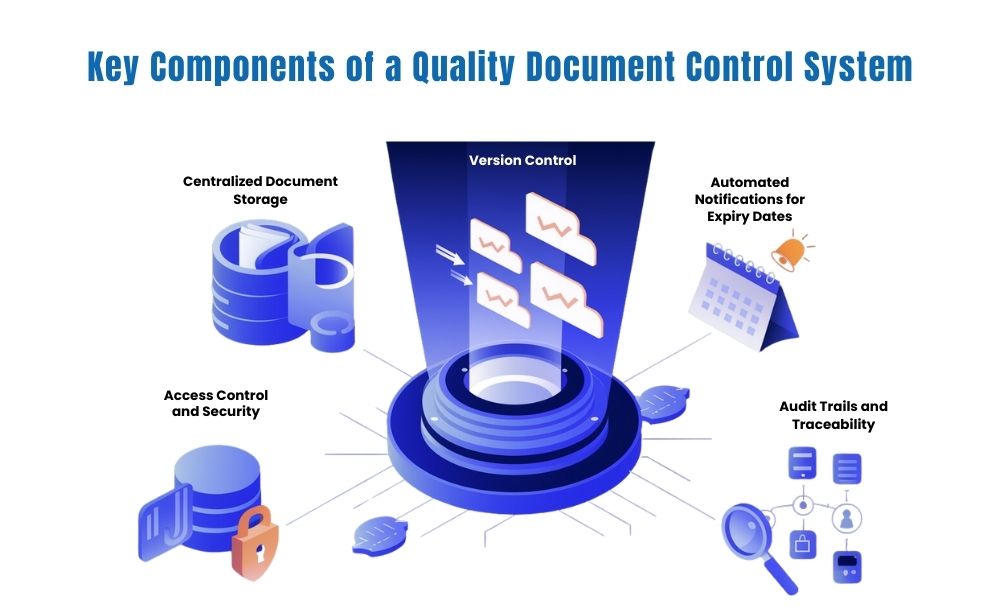
Key Components of a Quality Document Control System
A successful quality document control system includes several key components that help manage and track compliance efficiently. Here’s a closer look at what makes a system robust:
- Centralized Document Storage A centralized storage system is essential for organizing and accessing all supplier-related documents. By having a single source of truth for compliance documents, businesses can easily retrieve any supplier compliance document when needed.
- Version Control Accurate document control requires tracking revisions over time. With version control, businesses can ensure that they are always working with the latest versions of compliance documents, which reduces errors and ensures consistency.
- Automated Notifications for Expiry Dates Many compliance documents have expiration dates, and staying on top of these is crucial. Automated notifications for document expiration can prevent gaps in compliance and ensure that required documents are updated on time.
- Access Control and Security Protecting sensitive supplier information is critical. A quality document control system allows administrators to set permissions, so only authorized personnel can view or edit certain documents. This ensures data security and integrity.
- Audit Trails and Traceability An audit trail provides a record of document access, changes, and approvals. This feature supports transparency and helps companies comply with regulations, as it shows a clear history of document updates and interactions.
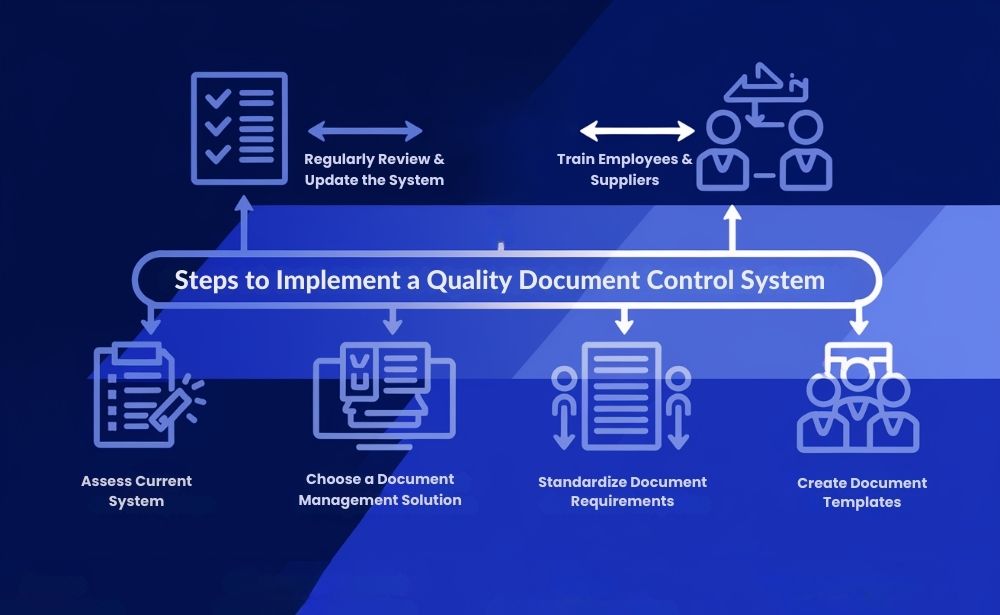
Steps to Implementing a Quality Document Control System for Supplier Compliance
Implementing a quality document control system tailored to supplier compliance can feel daunting, but following a structured approach makes it manageable. Here’s a step-by-step guide to help businesses get started:
- Assess Your Current System Start by evaluating your existing document management practices. Identify gaps, such as missing compliance documents, outdated information, or weak tracking systems. This assessment will help clarify what’s needed in a new document control system.
- Choose a Document Management Solution Select a solution that fits your business’s compliance needs. Look for features like automated reminders, version control, and centralized storage to manage your supplier compliance documents effectively. A platform like AsterDocs, designed for compliance-intensive industries, can provide robust tools for managing supplier documentation.
- Standardize Document Requirements Determine the specific documents required for each supplier. This might include certificates of analysis (COA), material safety data sheets (MSDS), and other regulatory documents. Establishing standard requirements ensures consistency and simplifies the document collection process.
- Create Document Templates Templates save time and maintain consistency across supplier documents. Consider creating templates for standard documents like supplier agreements, quality assurance checklists, and compliance certifications.
- Train Employees and Suppliers A quality document control system is only effective if it’s used correctly. Training employees and suppliers on best practices in document management, including how to update, access, and share documents, helps maintain compliance.
- Regularly Review and Update the System Compliance regulations and business needs evolve over time. Conduct regular reviews of your document control system to ensure it meets current standards and reflects changes in supplier requirements.
The Role of Quality Document Control in Supplier Quality Management
Supplier quality management goes beyond basic compliance; it involves assessing suppliers on quality, reliability, and adherence to industry standards. Quality document control is at the heart of this process. Here’s how it supports effective supplier quality management:
- Enhanced Visibility into Supplier Performance A quality document control system gives businesses a detailed view of each supplier’s compliance status. This includes tracking the completion of required documents, identifying gaps in compliance, and monitoring updates in real-time.
- Proactive Risk Mitigation By staying on top of document expiration dates, compliance updates, and certifications, businesses can proactively address issues before they impact the supply chain. According to a survey by Deloitte, 65% of companies that focus on proactive supplier management see a reduction in compliance risks.
- Facilitates Informed Decision-Making With detailed documentation and version history, businesses can make informed decisions about continuing or terminating supplier relationships. Companies with clear visibility into supplier compliance documents and performance metrics are better equipped to maintain quality standards across the board.
Benefits of Implementing Quality Document Control for Supplier Compliance
- Reduced Compliance Risks With a quality document control system, businesses are less likely to miss critical compliance updates, minimizing the risk of regulatory penalties.
- Improved Audit Readiness Being audit-ready is a huge advantage in regulated industries. By maintaining an organized system with all supplier compliance documents, businesses can navigate audits efficiently and with confidence.
- Increased Operational Efficiency Automated notifications and centralized storage make document retrieval quick and hassle-free, saving time and reducing administrative work.
- Better Supplier Relationships Clear documentation and open communication build trust. Suppliers appreciate transparency, and a well-structured document control system shows a commitment to quality, fostering stronger partnerships.
- Cost Savings While implementing a document control system requires an initial investment, the long-term savings in terms of reduced compliance fines, operational efficiency, and minimized risk of recalls are substantial.
AsterDocs: Simplifying Quality Document Control for Supplier Compliance
AsterDocs offers a comprehensive solution for businesses looking to streamline their quality document control practices. Here’s how AsterDocs supports supplier compliance:
- Centralized Document Library: Easily store, access, and organize supplier compliance documents in one secure location.
- Automated Version Control and Expiry Alerts: Never miss an update with automated notifications and version control.
- Document Traceability: Track document history, changes, and approvals for complete transparency.
- Secure Access Control: Ensure that only authorized personnel have access to sensitive documents, maintaining data integrity.
With tools designed for regulated industries, AsterDocs empowers businesses to take control of their supplier compliance and improve supplier quality management.
Conclusion
In today’s regulatory landscape, quality document control is not just a necessity—it’s a strategic asset. By implementing a well-structured document control system, businesses can enhance their supplier compliance efforts, strengthen relationships, and reduce compliance risks.
A quality document control system like AsterDocs supports all aspects of supplier quality management by making it easier to track, update, and manage critical compliance documents. For companies that prioritize compliance, efficiency, and long-term success, investing in AsterDocs can be an invaluable step.